Bohr could not give the reason of whole number of n in angular momentum
mvr = nh/2π equation
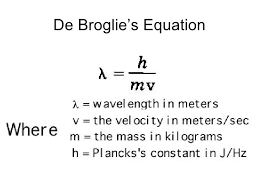
de- Broglie assumed that in the phenomenon of interference or diffraction the distance covered by electron should be integral multiple of wavelength (λ) . Since electron moves in circular path so it can move in only in those orbits whose circumference is some integral multiple of wavelength. Therefore,
2πr=nλ ……………….(a)
or 2πr =n.h/mv
or mvr = nh/2π …………………(b)
Above equation (b) is nothing but the Bohr’s hypothesis for circular motion of electron. Thus de-Broglie gave logical explanation of Bohr’s concept Fig. gives the Bohr’s and de-Broglie’s circular paths of electron. If nλ (5 λ) is a whole number, only then eaves will be in phase (fig. a), otherwise they will not be in phase (fig.b) in which n is not whole number. It means orbit (fig. b) is not allowed.

Conclusions from de-Broglie’s Equation : Following conclusions may be drawn from the de-Broglie’s equation .
de-Broglie’s wavelength λ is significant only for very minute particles like electron , photon etc. It is not significant for heavier particles for which mass (m) is very large so wavelength will be almost negligible (since λ = 1/m)
Wavelength is inversely proportional to the velocity of the particle Thus; wavelength for a stationary particle is indeterminate.
Wavelength does not depend upon the nature and charge of particle.
De-Broglie’s wave (matter waves) are entirely different from electromagnetic waves).